Approved! WEGO's egg retrieval solution has been approved for NMPA Class III registration certificate
WEGO egg retrieval fluid and WEGO assisted reproductive culture medium add another certificate
Recently, WEGO's independently developed series of assisted reproductive culture media products have received another certification, and the Follicle Flush Buffer [CFDA(ZZ)20233181892] has officially obtained the Class III Medical Device Registration Certificate issued by the National Medical Products Administration.
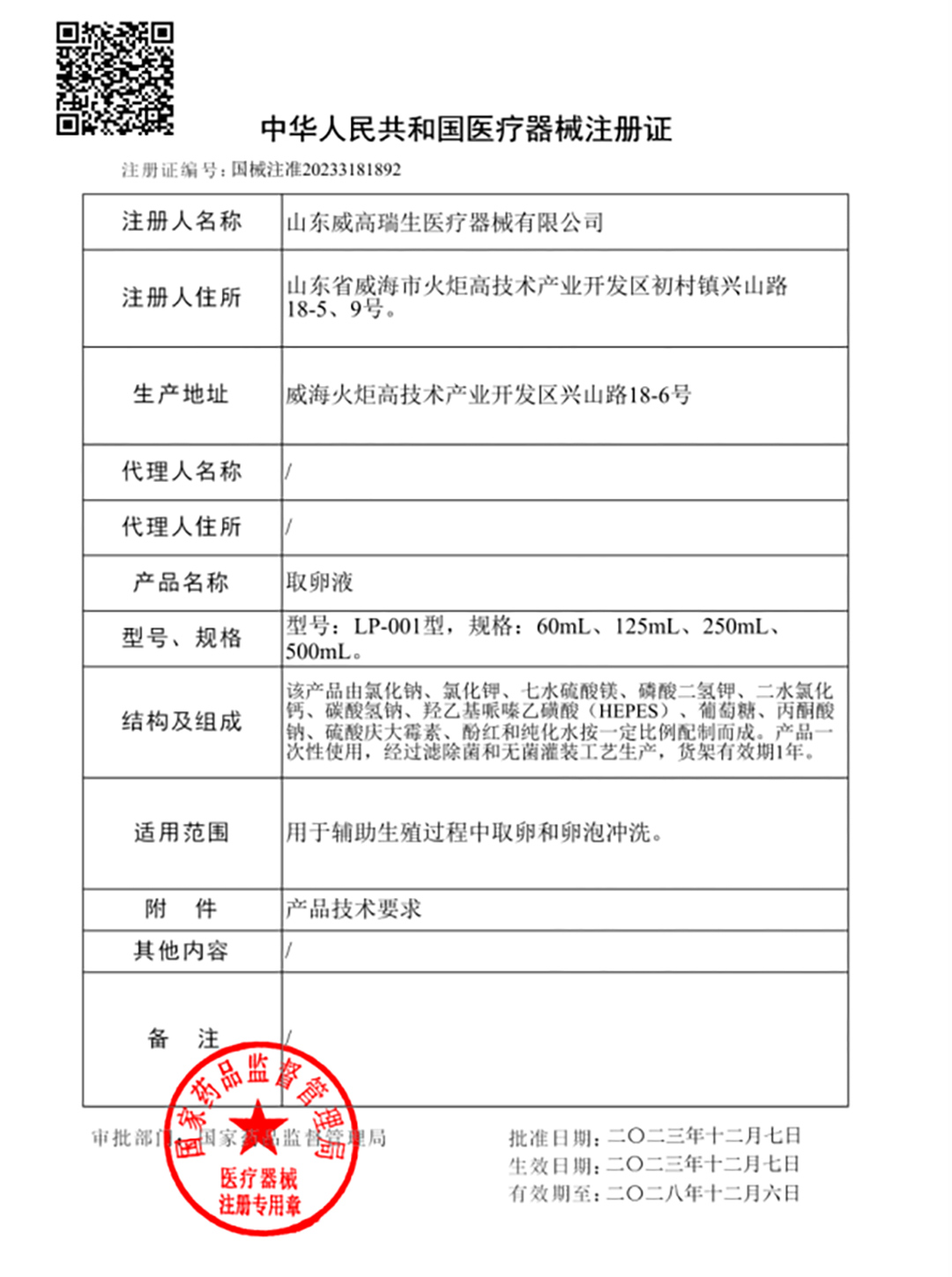
Previously, WEGO Reproductive had obtained NMPA Class III registration certificates for four products: Vitrification Kit [CFDA(ZZ)20233180341], Warming Kit [CFDA(ZZ)20233180372], Sperm Washing Medium [CFDA(ZZ)20233180343], and hepes-HTF [CFDA(ZZ)20233180345].

Recently, the China Medical Forum Newspaper launched the “Protecting Women's Health -2023 Assisted Reproductive Technology Tour” activity based on the “Navigation Plan”. Professor Li Rong, the chairman of the conference, pointed out in her speech that the number of ART treatments in China has exceeded one million egg retrieval cycles, and nearly 300000 newborns are born through ART every year, accounting for 3% -4% of the total number of births and leading the world.

How to promote the development of assisted reproductive technology from a product perspective and meet the high expectations of “improving treatment outcomes” in the face of rapidly growing market demand related to people's health and well-being has become a key concern for industry insiders.
WEGO Reproductive is committed to becoming a leader in providing valuable solutions in the field of reproductive health. Based on independent research and development, we have always cooperated sincerely with renowned assisted reproductive technology experts and research institutes at home and abroad, continuously focusing on the research and development, production, sales, and operation of reproductive centers for assisted reproductive products. We maintain a stable frequency of new releases and iterations, and currently have 7 consumables products, 5 culture media products, and 2 equipment products on the market, achieving full product supply.
With the gradual deepening of research and the increasing maturity of technology, it has been proven that using safe and mild reagents for assisted IVF technology can effectively improve survival rates. How to make reagents safer and gentler? WEGO believes that only with the spirit of craftsmanship and strict quality control can we achieve high-quality standards for fresh culture solution products.
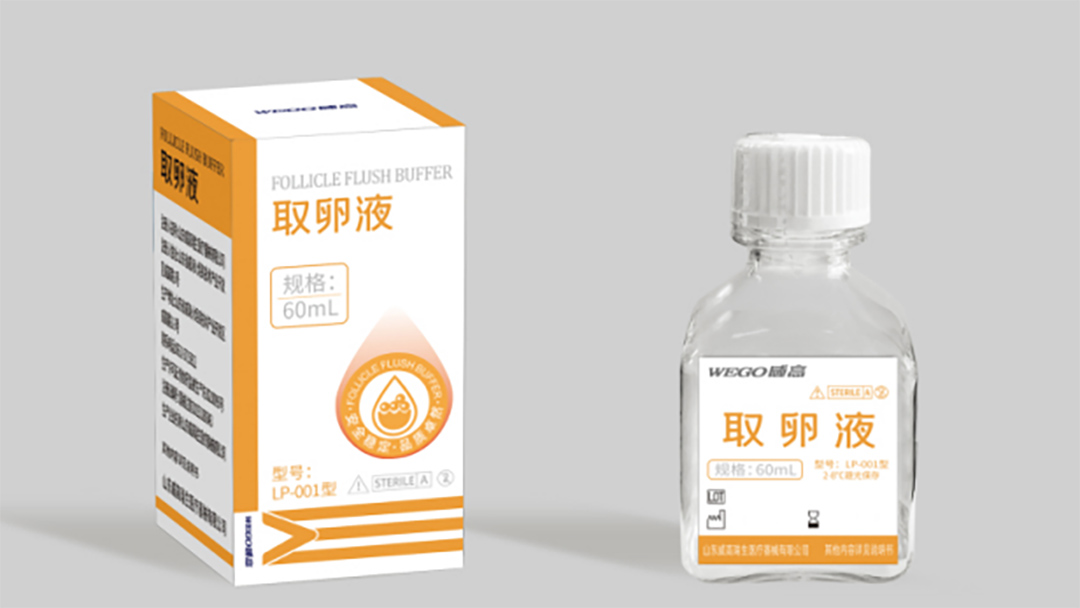
WEGO Follicle Flush Buffer is suitable for follicular flushing and oocyte collection in assisted reproduction, helping to maintain the quality of oocytes before fertilization, increase the number of oocyte complexes, and improve in vitro fertilization outcomes for patients with poor response to ovarian stimulation.
As a member of the WEGO assisted reproductive culture fluid series, the research and production of egg retrieval fluid adhere to consistent high-quality standards, rigorous scientific verification, and professional personnel operation are indispensable.
WEGO is the first brand in China to embark on the development of assisted reproductive culture media. Since the launch of mouse embryo experiments, the culture media research and development project team has twice dispatched personnel to study mouse embryo experiments at the Guangzhou Institute of Biomedical and Health Sciences and the Institute of Zoology, Chinese Academy of Sciences. They continuously optimize the operation process of mouse embryo experiments, update experimental micro operation tools, and explore more stable and reliable technical methods.
The employees of the company's experimental animals must hold certificates to work, effectively ensuring that the fresh food and accommodation conditions of experimental animals, as well as the health monitoring and epidemic prevention during feeding, meet the standards, and establish detailed and standardized operating procedures for in vitro mouse embryo testing and experimental animal feeding standards. In recent years, under the guidance of Dr. Wang Weihua, Chief Scientist of WEGO, the in vitro mouse embryo quality control of WEGO's assisted reproductive products has become more stringent and standardized, further consolidating the foundation for product safety.

Developing lean liquid formulas, strictly controlling quality standards, producing fresh liquids through order based production and cold chain transportation, together achieving high-quality standards for WEGO Follicle Flush Buffer.

I hope that the WEGO assisted reproductive series culture medium can become a reliable assistant for experts in the reproductive center laboratory, contributing its brand strength to the development of assisted reproductive industry!
- Previous:Full Line Product Promotion | WEGO Reproductive Products Debut at the 16th National Conference on Reproductive Medicine
- Next:Already the last article





 Address:No. 1, WEGO Road, Torch High-tech Industrial Development Zone, Weihai City, Shandong Province
Address:No. 1, WEGO Road, Torch High-tech Industrial Development Zone, Weihai City, Shandong Province Customer service hotline: 400-646-6666
Customer service hotline: 400-646-6666 Consultation hotline: 0631-5715912
Consultation hotline: 0631-5715912
