WEGO takes another solid step towards the planning of the entire line of assisted reproductive products!
Recently, the “Time-lapse Incubator” and “Disposable Embryo Culture Dish” developed by Shandong Wego Ruisheng Medical Device Co., Ltd. have both obtained medical device registration certificates [Registration Certificate No.20232180598; Registration Certificate No.20232180586], marking a solid step forward for WEGO's full line of assisted reproductive product planning and opening the era of intelligent cultivation in the field of WEGO's assisted reproductive technology.

Strong collaboration, creating high-quality products with “intelligence”
As one of the earliest medical device companies in China to enter the assisted reproductive industry, obtain the registration certificate for domestic assisted reproductive medical consumables, and become a writer of industry standards, WEGO always pays attention to the forefront of the industry trends and focuses on researching and developing new products and producing high-quality products.
As the most advanced cultivation equipment for in vitro fertilization, the Time-lapse Incubator is mainly composed of a temperature control system, a gas supply control system, a time difference shooting system and other host structures and system software. It can achieve the full process of embryo cultivation from fertilized egg to blastocyst stage, and through high-definition imaging system for online continuous observation, capture the morphological and dynamic changes of embryos during development, and automatically generate dynamic images for embryologists to observe. The entire cultivation process does not require opening the cultivation chamber to ensure the most stable growth environment for embryos.

In order to better serve and meet the technological iteration and customer needs in the reproductive field, WEGO has teamed up with the Chinese Academy of Sciences and internationally renowned embryologists to create high-quality products. Relying on professional technical support, WEGO Time-lapse Incubator and its dedicated dish have achieved innovative designs in the cultivation system, imaging system, gas supply system, and concentration dynamic adjustment. The microscope motion system, cultivation chamber, and dish structure have all obtained patent certificates.
Starting from the heart, “anticipate” high-quality products
In order to achieve a stable, accurate, safe, and convenient operating experience, the Time-lapse Incubator has developed and innovated around the following six characteristics during the independent project stage, based on product functions pushed back from usage scenarios, combined with market research and customer visits to preset product effects:
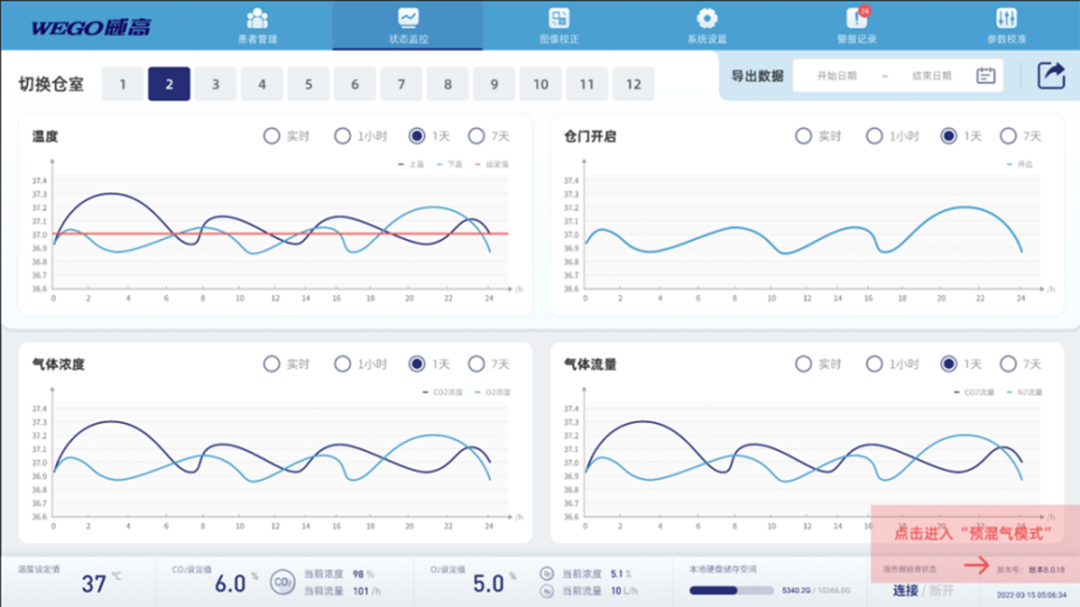
1. Human computer interaction: a good human-computer interaction interface, intuitive and easy to understand, with better user experience; The main interface is divided into six functional areas: “Patient Management”, “Status Monitoring”, “Image Correction”, “System Settings”, “Alarm Recording”, and “Parameter Calibration”.
2. Remote monitoring: It can remotely monitor the environmental information and cultivation situation of the incubator online, and can receive notification information with or without the internet. Even if you are in a different location, you can be familiar with the cultivation situation.
3. Independent chambers: 12 independent cultivation chambers can ensure a stable cultivation environment, with a maximum capacity of 16 embryos per chamber and independently set cultivation parameters. The shooting cycle can be customized, and the shooting cycle for full chamber cultivation is less than 10 minutes.

4. Precise temperature control: The temperature control is precise and uniform, and the cultivation chamber has the functions of heating the upper and lower covers simultaneously and controlling the temperature separately to prevent condensation. If the set temperature is exceeded, the system will automatically power off and issue an alarm signal. The over temperature protection function effectively protects the embryo cultivation temperature, making the cultivation process safer and more reassuring.
5. Optional gas supply mode: Supports premixed gas and three gas modes, which can be set according to customer needs. Each warehouse is equipped with independent temperature and gas concentration dynamic curve charts, which can be set by hours, days, 7 days and other time periods for easy observation at any time. The gas concentration control is precise and the deviation is small. Each chamber is equipped with an independent air pump and controlled independently to ensure that the gas concentration in the chamber meets the cultivation requirements.
6. Equipped with dedicated culture dishes: The dedicated time zone culture dish is designed with zoning, with a maximum of 8 embryos cultured in each zone. It effectively saves culture medium and oil when the number of embryos cultured is small. With the guidance of internationally renowned embryologists and strict physical, chemical, and biological performance testing, the embryo culture process is safer, more stable, and meets clinical requirements.
Seeing the big through the small, “tools” contain exquisite works
As the saying goes, “a glimpse reveals the whole leopard.” The Time-lapse Incubator is complex and intricate, and its precision and quality control standards can be explored from its professional accessories - Disposable Embryo Culture Dishs suitable for culturing oocytes and embryos in the WG-TL12 Time-lapse Incubator.
1、 Innovative structural design:
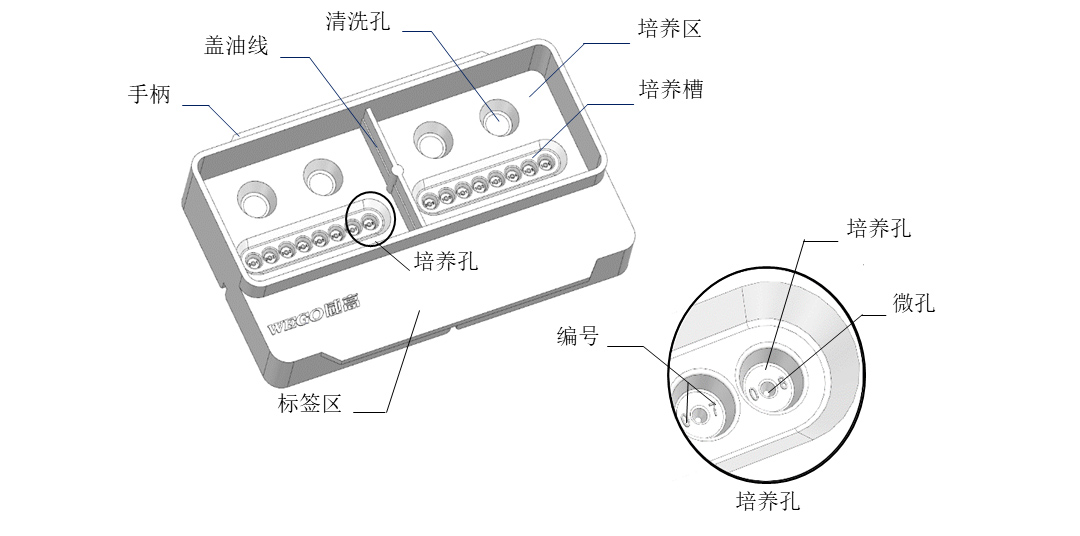
Guided by internationally renowned embryologists, the structure of the culture dish conforms to the usage habits and requirements of the reproductive laboratory. The culture area is specially designed for group culture and has anti misplacement functions to ensure operational safety and promote embryo growth.
① The design of dividing the culture dish into zones, with 8 culture wells per zone, a total of 16 culture wells and 4 cleaning wells, can effectively save the amount of culture medium and oil used;
② The petri dish has a reference line for oil cover, making it easier to achieve precise oil cover;
③ Cultivate micropores with digital markers that can clearly identify the pore number under a microscope, without the need for visual observation, providing a better interactive experience;
④ The carefully designed dish lid not only protects the embryos and reduces liquid evaporation, but also ensures the exchange of gas between the dish and the chamber;
⑤ The protruding wing plate structure on both sides makes it easy for the index finger and thumb to grasp and operate, making the operation smoother and more convenient;
⑥ Reserved label pasting area, located outside the cultivation area, for pasting patient information labels or electronic labels, making it faster to identify patient information and reducing the impact of VOC gas generated by labels on embryos;
2、 Strict performance verification:
Each dish undergoes strict quality control from raw materials to production and processing to ensure the safety of embryo culture.
① The petri dishes are made of safe and non-toxic medical grade GPPS materials to ensure product safety from the source;
② The culture dish has been validated through in vitro cytotoxicity, pyrogen, and other biological and chemical tests to ensure its non toxicity and absence of the product's own heat source;
③ The mouse embryo test has been tested by a professional organization and meets the requirement of a blastocyst formation rate of ≥ 80%, further verifying the safety of cultured embryos;
④ The requirements for bacterial endotoxins are stricter, and after professional testing, the bacterial endotoxins are less than 1EU/set;
⑤ After irradiation sterilization, the sterile assurance level is SAL10-6 or even better, making it a disposable product.
⑥ The surface of the culture dish has been specially treated to reduce the phenomenon of embryo wall attachment and the generation of bubbles in the culture medium, which is more conducive to embryo manipulation.





 Address:No. 1, WEGO Road, Torch High-tech Industrial Development Zone, Weihai City, Shandong Province
Address:No. 1, WEGO Road, Torch High-tech Industrial Development Zone, Weihai City, Shandong Province Customer service hotline: 400-646-6666
Customer service hotline: 400-646-6666 Consultation hotline: 0631-5715912
Consultation hotline: 0631-5715912
